Are you or a loved one navigating the challenges of Parkinson’s Disease while trying to stay safe behind the wheel? Driving represents freedom and independence, but when Parkinson’s enters the equation, it brings a host of questions and concerns. How does Parkinson’s affect driving ability? What assessments can ensure safety on the road? Dive into our comprehensive guide, where we unravel the mysteries of driving with Parkinson’s, from essential evaluations to adapting for safety. Stay tuned as we steer through the turns and traffic lights of Parkinson’s and driving, ensuring you’re equipped to navigate this journey with confidence and care. These are primarily created based upon a recent systematic review in Movement Disorders journal (2024 March).
- How Common Are Driving-Related Issues Among Individuals with Parkinson’s Disease?
Driving-related issues are relatively common among individuals with Parkinson’s Disease (PD), significantly affecting their independence and quality of life. The article highlights that a meta-analysis found PD patients, especially those with an average disease duration of 6.7 years, are more likely to fail an on-road driving test and have over a two-fold increase in crash risk in driving simulator tests compared to healthy counterparts. Additionally, it notes that PD patients exhibit a gradual deterioration in their driving abilities and tend to cease driving earlier than those without the condition.
The combination of PD’s motor symptoms (like bradykinesia, rigidity, and tremors), cognitive impairments (such as issues with executive functioning and spatial awareness), and the effects of medication (including drowsiness or sudden sleep onset) all contribute to the challenges faced by individuals with PD when driving. These factors underscore the importance of regular and comprehensive evaluations of driving fitness for people with PD to ensure safety on the road.
- Why Do People with Parkinson’s Disease Face Difficulties in Driving?
Individuals with Parkinson’s Disease (PD) encounter driving difficulties due to a combination of motor and non-motor symptoms, as well as the side effects of medications used to manage the condition. The motor symptoms include bradykinesia (slowness of movement), rigidity, rest tremor, and postural instability. These symptoms can impair physical abilities necessary for driving, such as steering, braking, and accelerating.
Non-motor symptoms that affect driving include cognitive impairments, which might involve challenges with attention, decision-making, and spatial awareness. Neuropsychiatric symptoms, such as depression and anxiety, can also impact driving abilities. Furthermore, sleep disorders associated with PD, like excessive daytime sleepiness, can make it dangerous to drive.
The medications prescribed for PD, while essential for managing symptoms, can have side effects like sudden onset of sleep, which poses a significant risk for driving. The complex interplay of these factors contributes to the driving difficulties experienced by people with PD, making it crucial to assess their driving fitness regularly.
- What are the potential risks for driving with Parkinson’s Disease?
Driving with Parkinson’s Disease (PD) comes with potential risks due to the symptoms of the condition and the side effects of medications used in its management. Here’s a simplified overview of these risks:
- Motor Skills Impairment: PD can cause tremors, stiffness, and slowness of movement, making it hard to steer, accelerate, or brake quickly when needed.
- Cognitive Changes: PD can affect memory, attention, and problem-solving skills, which are crucial for navigating, responding to unexpected events, and making split-second decisions on the road.
- Visual Disturbances: Some people with PD experience vision problems, such as difficulty with depth perception and contrast sensitivity, making it harder to see road signs, signals, and obstacles.
- Sudden Onset of Sleep: Medications for PD, especially dopamine agonists, can lead to sudden sleepiness or even sleep attacks, which can occur without any warning, posing a significant risk while driving.
- Fluctuating Symptoms: PD symptoms can fluctuate throughout the day, with periods of better or worse motor function. This unpredictability can affect driving abilities at different times.
- Impaired Reaction Time: PD can slow physical and mental reactions, delaying responses to traffic lights, other vehicles, pedestrians, or unexpected hazards.
Understanding these risks is essential for individuals with PD, their families, and healthcare providers to make informed decisions about driving. Regular evaluations and adjustments to driving habits or the decision to stop driving may be necessary to ensure safety.
- Do Countries Have Official Driving Guidelines for People with Parkinson’s Disease?
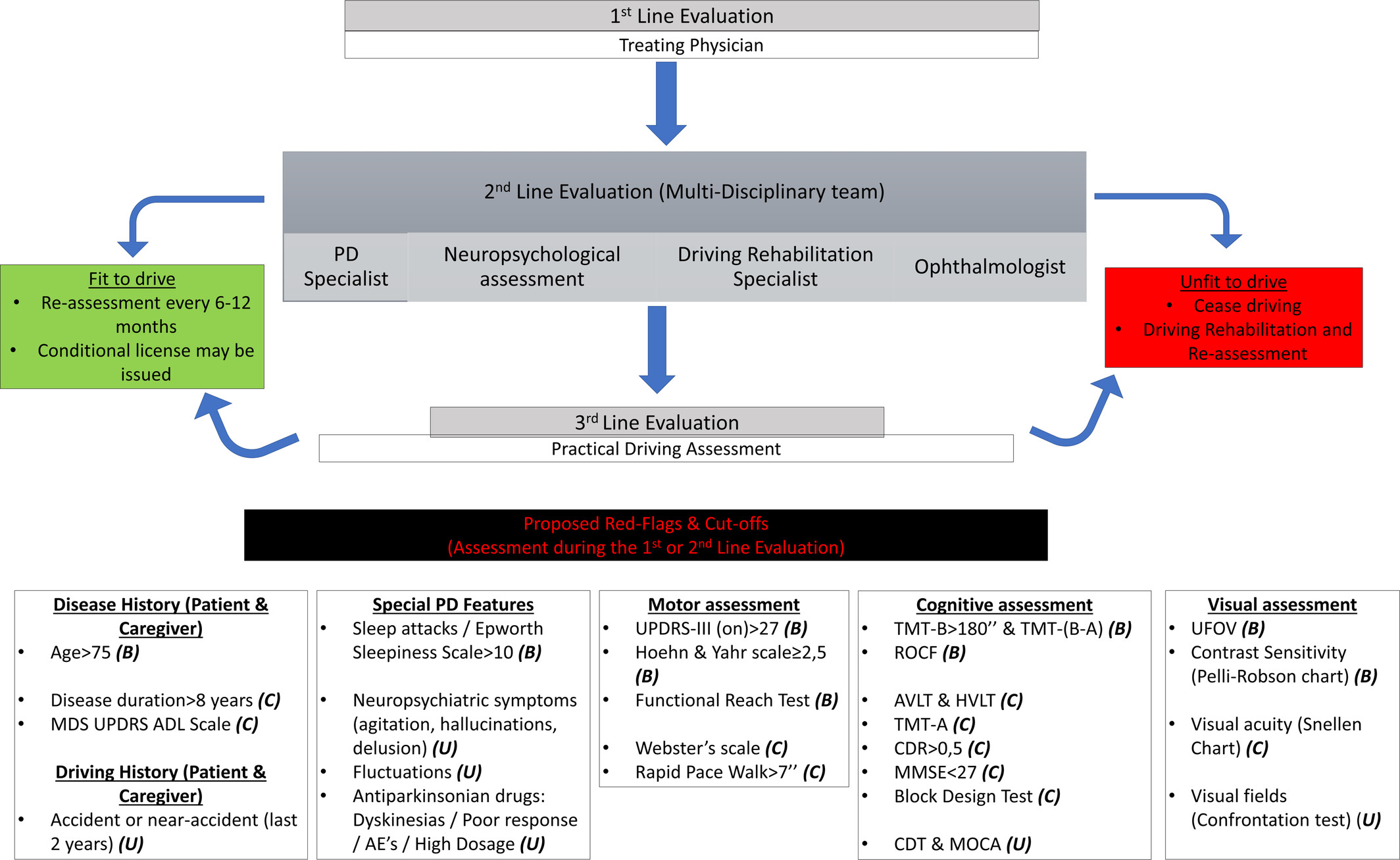
Yes, several countries have officially established guidelines for evaluating and managing the driving abilities of individuals with Parkinson’s Disease (PD). According to the systematic review covered in the article, nine national guidelines were identified from seven different countries. These countries are Australia, Canada (which has two separate sets of guidelines from different organizations), Ireland, New Zealand, Singapore, the United Kingdom, and the United States (also with two distinct guidelines from different entities). These guidelines aim to assess the fitness to drive of individuals with PD, considering the unique challenges posed by the condition.
- What Specific Tests Are Used in the Assessment of Driving Fitness for Parkinson’s Disease Patients?
In evaluating the driving fitness of individuals with Parkinson’s Disease (PD), several specific tests are recommended to comprehensively assess motor, cognitive, and visual abilities. These tests aim to determine a person’s capability to drive safely. Here’s a breakdown of these tests in layman’s terms:
- Motor Assessment Tests:
- Rapid Paced Walk Test (RPWT): This test checks how quickly and safely a person can walk a short distance. It helps understand the person’s mobility and balance, which are crucial for operating pedals and getting in and out of a car. (https://icsw.nhtsa.gov/people/injury/olddrive/safe/01c02.htm ) The RPWT is valuable because it is quick, easy to administer, requires minimal equipment, and can be performed in various settings. While it directly assesses walking ability, the insights gained can indirectly inform evaluations of driving fitness by indicating the level of physical function and mobility.
-
- Manual Tests of Motor Strength and Range of Motion: These involve simple exercises to assess the strength of arms and legs, and how well a person can move their joints. Such movements are vital for steering, turning, and using car controls.
- Cognitive and Neuropsychological Tests:
- Trail Making Test-B (TMT-B): This paper-and-pencil test involves connecting numbered and lettered dots in a specific order as quickly as possible. It evaluates a person’s ability to switch attention between tasks, a skill needed for keeping track of road conditions, navigation, and responding to unexpected events.
- Clock Drawing Test: In this test, the person is asked to draw a clock showing a specific time. It checks spatial awareness and the ability to plan and execute a task—key for understanding road signs and making turns.
- Mini-Mental State Examination (MMSE): This brief 30-point questionnaire assesses various cognitive functions, including arithmetic, memory, and orientation, indicating the overall cognitive ability that impacts decision-making while driving.
- Visual Assessment Tests:
- Visual Acuity Test: This test, often done using an eye chart, checks how clearly a person can see at distances, critical for reading road signs and seeing obstacles.
- Visual Fields Test: This evaluates the full horizontal and vertical range of what a person can see without moving their eyes, important for detecting vehicles, pedestrians, and other objects in peripheral vision.
- Contrast Sensitivity Test: This measures how well a person can distinguish between objects and their background, especially in poor light, fog, or glare, which affects night driving and driving under challenging weather conditions.
These evaluations are typically conducted in a clinical setting by healthcare professionals, including neurologists, occupational therapists, and sometimes driving specialists. The aim is to ensure that individuals with PD can meet the demands of safe driving or identify areas where adaptations might help. Regular reassessment is recommended to account for the progressive nature of PD and its impact on driving skills.
- What Are the Red Flags Indicating That a Person With Parkinson’s Disease May Not Be Fit to Drive?
For individuals with Parkinson’s Disease (PD), certain “red flags” signal that driving may no longer be safe. These indicators are critical for evaluating when it might be time to reassess driving abilities or consider stopping driving altogether. Here’s a simplified explanation of these warning signs:
- Motor Function Impairment: Difficulty with movements, such as stiffness or tremors, that could affect the ability to steer, brake, or accelerate smoothly.
- Cognitive Decline: Issues with memory, attention, problem-solving, or multitasking that impair the ability to navigate, respond to road signs, or make quick decisions in traffic.
- Visual Impairments: Problems with seeing clearly, judging distances, or having a limited field of vision, making it hard to spot vehicles, pedestrians, or obstacles.
- Increased Reaction Times: Slower responses to unexpected events, such as needing to brake suddenly or react to a traffic signal change.
- Medication Side Effects: Experiencing sudden sleepiness, dizziness, or other effects from PD medications that could impair driving at any moment.
- History of Close Calls or Minor Accidents: An increase in “near misses,” fender benders, or trouble with parking could indicate declining driving skills.
- Feedback from Others: Concerns expressed by family members, friends, or others about the individual’s driving performance or safety.
- Feeling Anxious or Overwhelmed While Driving: Increased stress or discomfort when driving, especially in complex situations like heavy traffic or unfamiliar areas.
- Difficulty with Driving Tasks: Problems with tasks that used to be easy, such as making turns, merging onto highways, or maintaining lane position.
- Navigational Challenges: Getting lost, even in familiar areas, or difficulty following directions due to cognitive decline.
Recognizing these red flags is crucial for ensuring the safety of the driver with PD, their passengers, and others on the road. Regular assessments by healthcare professionals can help monitor these factors and make informed decisions about driving fitness.
- How should a person with Parkinson’s Disease evaluate for his driving fitness?
Here’s a simplified explanation of how someone with Parkinson’s Disease (PD) should go about testing for driving fitness, :
- Start with Your Doctor: The first step is to talk to the doctor treating your PD, usually a neurologist. They know your health history and how PD affects you, making them a good starting point for evaluating your driving fitness.
- Check Your Physical Abilities: You might be asked to perform certain physical tasks to see how well you can move. This could include walking quickly or showing how strong and flexible your arms and legs are. These tests help determine if you can control a car safely.
- Assess Your Thinking Skills: Since driving requires quick thinking and problem-solving, your doctor might also check your cognitive abilities. This could involve tests where you connect dots, draw a clock, or remember lists of words. These tests check your ability to pay attention, make decisions, and remember important information while driving.
- Evaluate Your Eyesight: Good vision is crucial for driving, so your eyesight will be checked. This can include reading letters from a distance (like a standard eye chart test), checking your peripheral vision, and perhaps assessing how well you see contrasts, which is important for driving at night or in poor weather.
- Consider PD Symptoms and Medication Side Effects: Your doctor will think about how your PD symptoms and the side effects of your medication might affect your driving. For example, if your medication makes you drowsy, this is important to consider.
- Undergo Specialized Driving Tests if Needed: Based on these evaluations, your doctor might suggest a specialized driving test. This can be a practical test in a car to see how well you handle actual driving situations.
- Follow-Up Tests: Since PD can change over time, you might need to go back for regular check-ups to make sure you can still drive safely.
- Make Adjustments as Needed: If the tests show that driving could be risky for you or others, your doctor might suggest ways to adjust. This could mean driving only during the day, using special equipment to make driving easier, or exploring alternatives to driving.
In simple terms, testing for driving fitness in PD involves a combination of medical evaluations, physical and cognitive tests, and practical driving assessments, all aimed at ensuring you can drive safely without putting yourself or others at risk.
- Where can someone read about ‘Fitness for Driving’ in Parkinson’s Disease?
Here’s a suggested list of resources and types of documents where you can learn more about driving guidelines for people with Parkinson’s Disease:
- National Guidelines: Look for guidelines issued by national health or transportation authorities in various countries, such as Australia, Canada, Ireland, New Zealand, Singapore, the United Kingdom, and the United States. These guidelines provide country-specific recommendations for evaluating driving fitness in individuals with PD.
- Recommendation Papers from Professional Associations: Papers published by associations like the American Academy of Neurology (AAN) often provide evidence-based recommendations for clinicians assessing the driving capabilities of their patients with PD.
- Consensus Statements: Documents like consensus statements from expert panels offer agreed-upon guidance based on the latest research and expert opinion. These can help in understanding the collective stance on driving assessment procedures and criteria for people with PD.
- Research Studies on Driving and PD: Academic journals and medical research platforms often publish studies on the effects of PD on driving, evaluation methods, and intervention outcomes. These studies can provide data-driven insights into the challenges and solutions related to driving with PD.
- Resources from Parkinson’s Disease Foundations and Associations: Organizations dedicated to PD support and research, such as the Parkinson’s Foundation, Michael J. Fox Foundation for Parkinson’s Research, and Parkinson’s UK, may offer resources, guides, and articles on driving with PD.
- Government and Transportation Department Websites: Many countries’ transportation or road safety departments provide guidelines and resources for drivers with medical conditions, including PD. These resources can offer practical advice and legal considerations for driving with a health condition.
- Occupational Therapy and Driving Rehabilitation Resources: Organizations specializing in occupational therapy and driving rehabilitation may offer resources on adaptations, evaluations, and training programs to support safe driving among individuals with PD.
- Online Forums and Community Support Groups: Online platforms and social media groups for individuals with PD and their families can be a source of shared experiences, tips, and advice on managing driving and PD.
- How often should a person with Parkinson’s disease undergo a driving assessment?
For individuals with Parkinson’s Disease (PD), the frequency of driving assessments is not one-size-fits-all; it should be personalized based on the progression of their condition, the impact of symptoms on driving abilities, and any changes in treatment. The article suggests that regular follow-up assessments are recommended due to the progressive nature of PD. While a specific timeline isn’t universally mandated, the guidelines suggest a range that could be as frequent as every 6 months to as long as 5 years, depending on individual circumstances.
In practice, the treating physician, often a neurologist familiar with the patient’s condition, plays a crucial role in determining the assessment frequency. They will consider factors such as:
- The severity and progression rate of PD symptoms.
- The presence of any cognitive decline or visual impairment.
- The effects of PD medications on alertness and motor control.
- Feedback from the patient and family members about driving capabilities.
- Any recent incidents or near-misses while driving.
Given these variables, the decision on how often to undergo driving assessment should be made collaboratively between the individual with PD, their family, and their healthcare team. This ensures a balance between maintaining independence and ensuring safety on the road for all.
In navigating the journey of driving with Parkinson’s Disease, the road might seem uncertain, filled with caution signs and speed bumps. However, armed with the right knowledge, assessments, and adaptations, it’s possible to maintain independence and safety behind the wheel. Remember, each journey is unique, and staying in tune with your body, seeking regular evaluations, and making informed decisions are key to driving safely with Parkinson’s. As we conclude this guide, let’s embrace the journey ahead with caution, courage, and the confidence that comes from being well-informed. Safe travels!
Reading reference: Stamatelos, P., Economou, A., Yannis, G., Stefanis, L. and Papageorgiou, S.G. (2024), Parkinson’s Disease and Driving Fitness: A Systematic Review of the Existing Guidelines. Mov Disord Clin Pract, 11: 198-208. https://doi.org/10.1002/mdc3.13942