#KeepMovingForParkinson: A Progressive Exercise Guide for People with Parkinson’s Disease
Welcome to the official movement guide from the #KeepMovingForParkinson initiative – a curated set of 16 functional exercises, categorized across 4 progressive levels of physical activity. This program is not time-bound. Instead, it is designed for people with Parkinson’s Disease to choose exercises based on their current mobility level – from bed-bound to fully mobile.
Each level contains 4 exercises, with clear steps, benefits, and guidance on who should perform them. Whether you’re starting from bed or standing strong, these movements are designed to help you stay active, build confidence, and improve quality of life.
🛎️ Level 1: Bed-Based Exercises
For individuals with limited mobility
1. Bridging

Steps: Lie on your back with knees bent and feet flat. Slowly lift your hips upward. Hold for 10 seconds, lower back down. Benefits: Improves glute and core strength, reduces stiffness. Who Should Do It: Patients in bed rest or significant mobility limitation.
2. Butterfly Stretch

Steps: Sit or lie down, bring the soles of your feet together, knees out. Gently press knees toward the bed. Benefits: Enhances hip flexibility. Who Should Do It: Those with hip stiffness or reduced pelvic mobility.
3. Side-to-Side Leg Roll

Steps: Lie on your back with knees bent. Gently roll knees side to side. Benefits: Improves trunk rotation and spinal mobility. Who Should Do It: People with trunk stiffness or poor rotational movement.
4. Leg Raise

Steps: Keep one leg bent, other straight. Lift straight leg to 30 degrees. Hold, then lower. Benefits: Strengthens quads and core. Who Should Do It: Anyone needing lower limb activation from bed.
💺 Level 2: Chair-Based Exercises
For individuals able to sit upright with or without support.
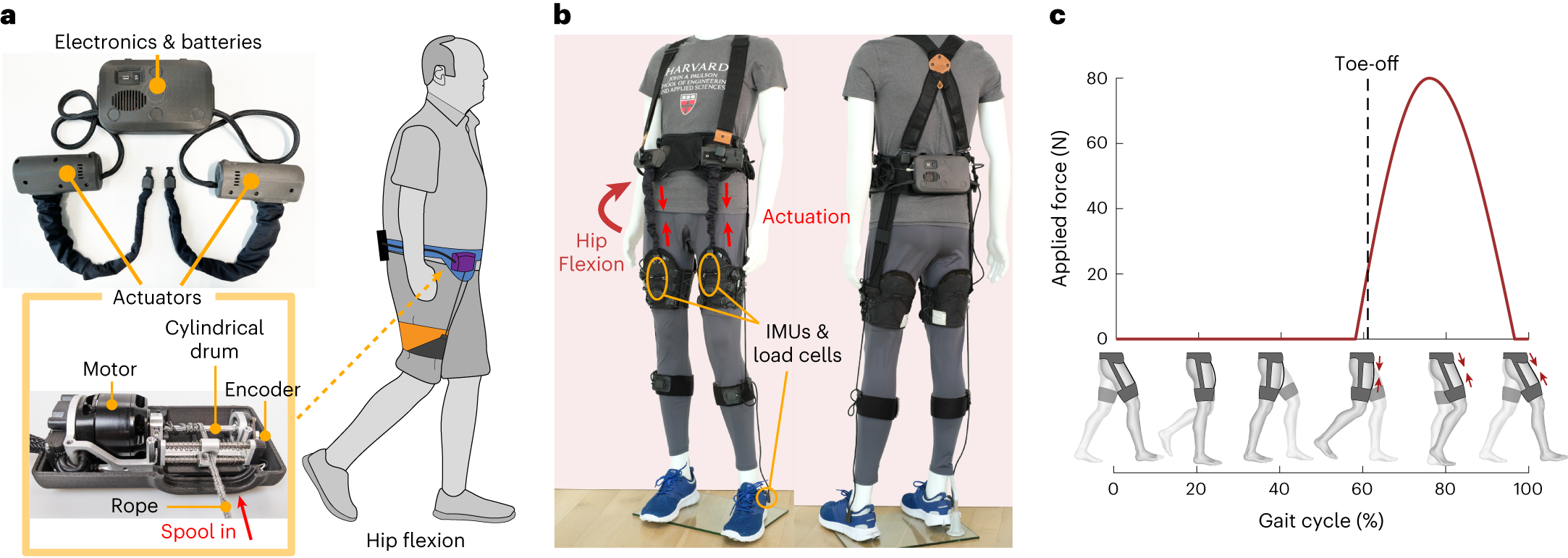
5. Trunk Rotation
Steps: Sit tall, arms crossed over chest. Gently rotate the trunk side to side. Benefits: Improves spinal mobility and trunk control. Who Should Do It: Those beginning seated rehab.
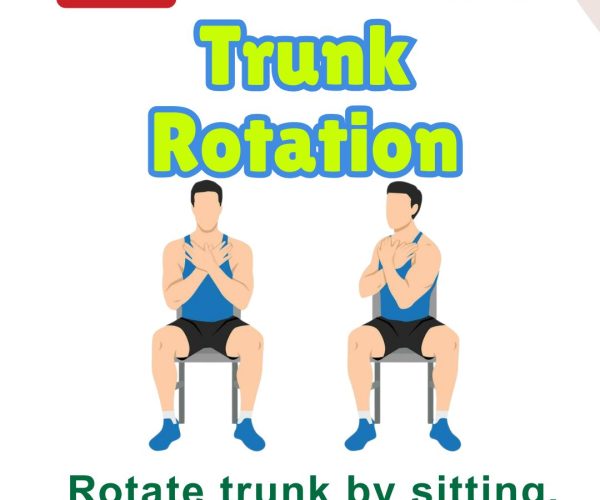
6. Hand Swing
Steps: Sit comfortably. Swing one arm forward and back rhythmically, then switch. Benefits: Promotes upper limb rhythm and arm swing. Who Should Do It: People with freezing or reduced arm movement.
7. Seated Marching

Steps: Lift one knee up at a time in a marching motion while seated. Benefits: Boosts leg coordination and circulation. Who Should Do It: Anyone preparing for standing transitions.
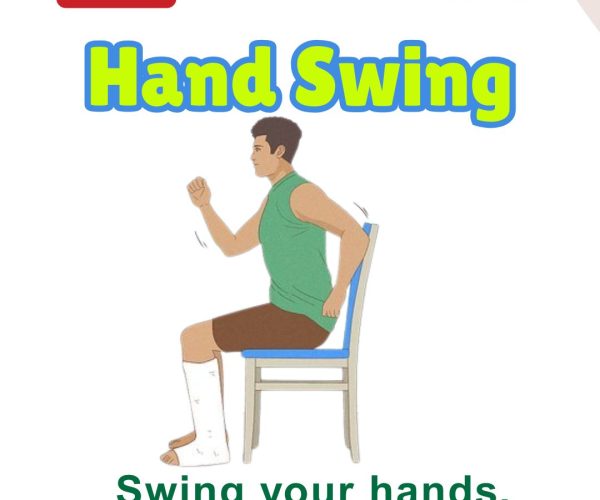
8. Sit to Stand
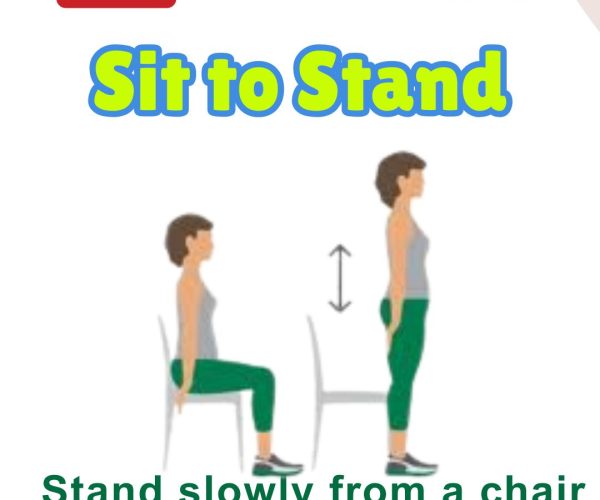
Steps: From seated position, rise to stand using as little hand support as possible. Benefits: Builds leg strength and functional independence. Who Should Do It: Ideal for improving transition from sitting to standing.
🧶 Level 3: Sit/Stand-Based Exercises
For individuals able to stand with support or partial independence.
12. Step with Support

Steps: Hold a chair for support. Step forward and back slowly. Benefits: Builds stepping confidence. Who Should Do It: Anyone practicing gait with balance limitations.
11. Step and Reach
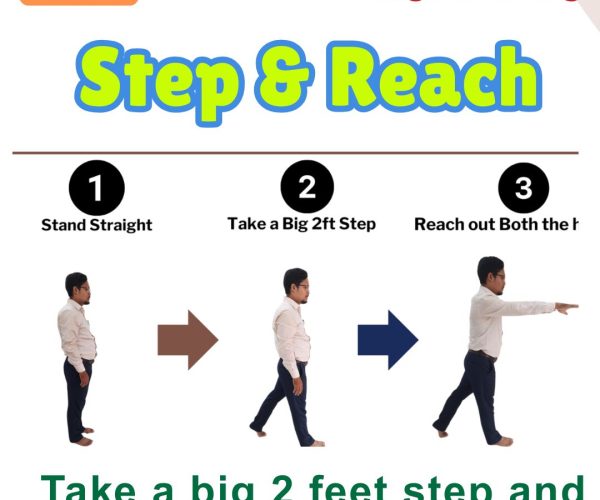
Steps: Take a 2-foot forward step, reaching arms forward. Benefits: Improves stride length and coordination. Who Should Do It: For those retraining functional walking.
10. Side-to-Side Reach

Steps: Stand or sit. Reach one arm to the opposite side, alternating slowly. Benefits: Enhances trunk flexibility and balance. Who Should Do It: Patients working on side stability.
9. Floor to Ceiling Reach
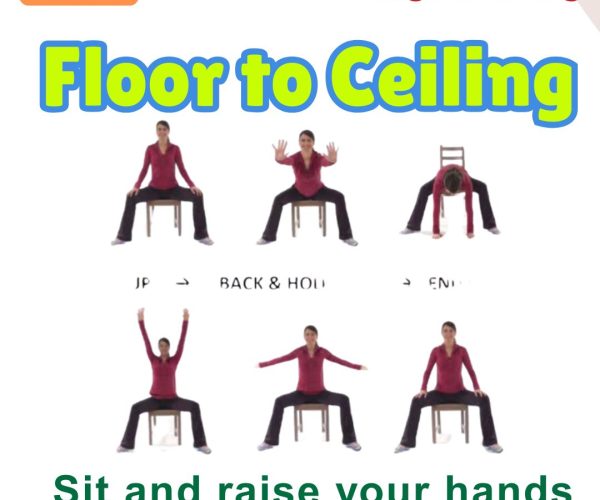
Steps: Start seated or standing. Reach arms down to the floor, then up to the ceiling. Benefits: Encourages full-body range of motion. Who Should Do It: Those with mild movement initiation chall
🏃️ Level 4: Standing and Dynamic Balance Exercises
For individuals with good standing balance or progressing toward full mobility.
15. Rock & Reach

Steps: Stand tall, swing arms sideways and rotate the body. Benefits: Activates large movements, improves balance. Who Should Do It: Great for those practicing functional trunk rotation.
14. Hurdle Crossing

Steps: Step over low objects spaced 1 foot apart (use support if needed). Benefits: Trains high stepping and obstacle avoidance. Who Should Do It: People with reduced foot clearance.
13. Big Step Walk

Steps: Take long, deliberate steps around a room (goal: 2000 steps daily). Benefits: Improves gait and step length. Who Should Do It: Those with shuffling or freezing gait.
16. One Leg Stance

Steps: Stand and lift one leg off the ground. Hold for 10–15 seconds. Alternate. Benefits: Enhances single-leg balance. Who Should Do It: Suitable for fall-prevention and high-level balance training.
Final Notes
Consult your doctor or physiotherapist before starting new exercises.
Modify based on fatigue or stiffness.
Repeat exercises daily or on alternate days based on tolerance.