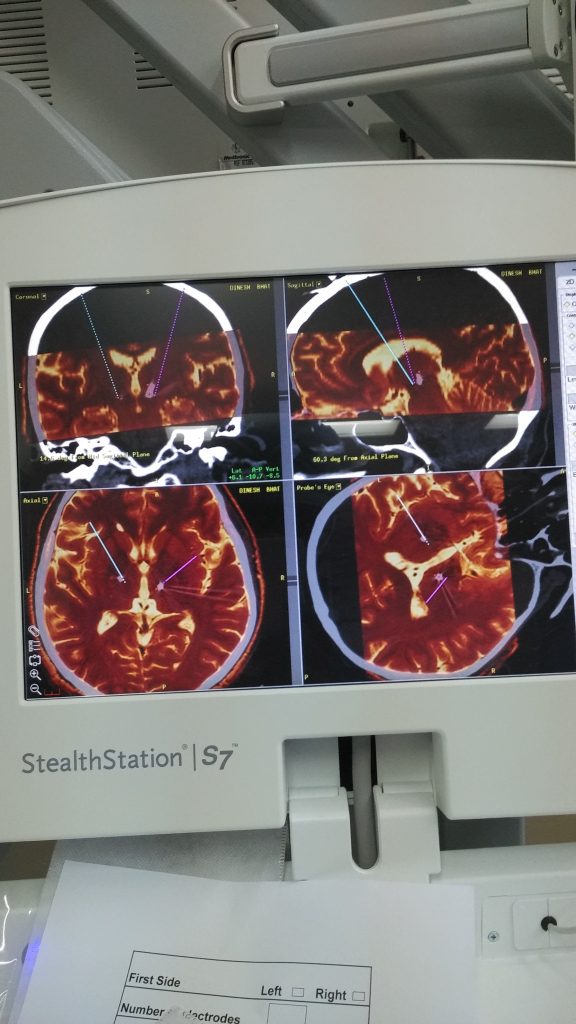
CyberKnife Radiosurgery Explained: Clarifying the 15-Minute Parkinson’s Procedure in the News
A recent news article highlighted a 78-year-old Parkinson’s disease patient whose life reportedly changed after a 15-minute CyberKnife radiosurgery session. Are you wondering how this procedure works, how it compares to other lesioning surgeries, or who might benefit from it?

In this video, we clarify the details behind the headlines (Cyberknife Radiosurgery for Parkinson’s) :
-
What is CyberKnife radiosurgery and what are Lesioning surgeries for the Brain?
- Understanding this noninvasive, high-precision radiation treatment.
- Also understanding various types of Brain Lesioning surgeries – Advantages and Limitations
-
How can it help Parkinson’s patients?
- The mechanism behind targeting specific brain areas to reduce tremor and other movement symptoms.
-
Comparing different lesioning approaches
- Focused Ultrasound (FUS), Gamma Knife, Radiofrequency ablation, and Deep Brain Stimulation (DBS)—what’s the difference?
-
Important considerations
- Pros and cons, potential side effects, time to see results, and long-term follow-up.
We created this presentation to answer common questions from patients, caregivers, and anyone curious about newer treatments for Parkinson’s disease. This is not a replacement for medical advice; we encourage you to consult a movement disorder specialist or neurosurgeon to see if radiosurgery is right for you.
1. What Are Lesioning Surgeries?
Lesioning surgeries involve the use of techniques (chemical, radiofrequency, focused ultrasound, or even stereotactic radiosurgery) to create a precisely targeted lesion in a specific brain area thought to be responsible for a particular set of symptoms. By destroying these targeted cells or interrupting pathological circuit activity, symptom relief can often be achieved.
Common Techniques for Creating Lesions
- Radiofrequency Lesioning: A probe is inserted stereotactically, and high-frequency currents produce heat that destroys the target tissue.
- Focused Ultrasound (FUS): Uses high-intensity ultrasound waves focused on a specific area in the brain without needing an incision, guided by MRI.
- Stereotactic Radiosurgery (e.g., Gamma Knife): Uses focused radiation beams to lesion the target gradually.
- Chemical Lesioning (much less common today): Injecting chemicals that destroy local neurons.
2. Historical Context
-
Early Psychosurgery (e.g., Lobotomies)
- In the early to mid-20th century, surgeries like the prefrontal lobotomy were used to treat psychiatric disorders, often with severe and unpredictable side effects. Because of ethical concerns and poor patient outcomes, these procedures largely fell out of favor.
-
Movement Disorders
- In the mid-20th century, lesioning of the thalamus (thalamotomy) and the globus pallidus (pallidotomy) became accepted treatments for movement disorders such as Parkinson’s disease.
- The success of these procedures opened the door to more targeted surgeries, aided by advances in imaging and stereotactic neurosurgical techniques.
-
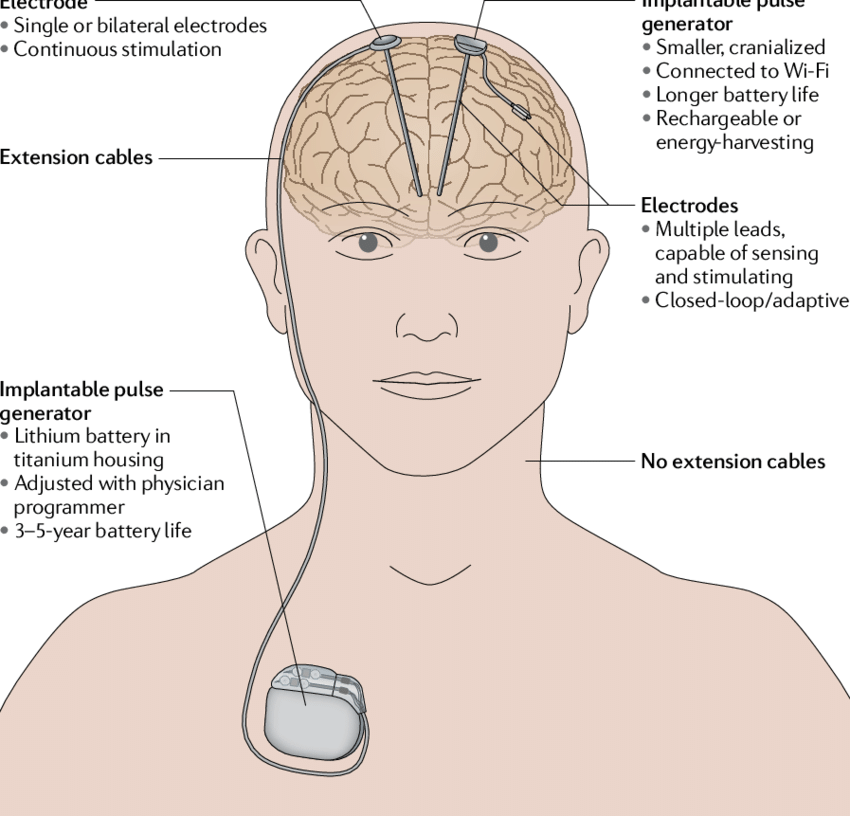
Shift to Deep Brain Stimulation (DBS)
- Starting in the 1990s, DBS began to replace many ablative procedures. Unlike lesioning, DBS uses an implanted electrode to modulate (rather than destroy) the function of specific brain areas. The effect is adjustable and reversible, which is a major advantage over lesioning.
3. Modern Clinical Indications
Today, lesioning surgeries are still performed, though less frequently than DBS. Some of the conditions for which lesioning may be considered include:
-
Parkinson’s Disease (PD)
- Pallidotomy: A lesion is made in the globus pallidus internus (GPi) to reduce symptoms such as tremor, rigidity, or dyskinesias.
- Subthalamotomy: Targets the subthalamic nucleus (STN), primarily to address Parkinsonian tremors and rigidity (though more commonly, STN DBS is performed).
-
Essential Tremor
- Thalamotomy (usually the ventral intermediate nucleus, or VIM, of the thalamus) can effectively reduce tremor.
- Focused Ultrasound Thalamotomy has gained traction as a noninvasive approach for essential tremor, approved in some countries for patients who are DBS-ineligible or prefer a less invasive option.
-
Dystonia
- Lesions in the globus pallidus internus or other basal ganglia structures might help reduce dystonic movements, although DBS is again more common.
-
Obsessive-Compulsive Disorder (OCD)
- In very severe, treatment-refractory cases, certain focused lesion surgeries (such as cingulotomy) have been tried. However, psychosurgical lesioning is highly regulated and usually considered only after extensive medical and psychological treatment fails.
4. Advantages and Disadvantages
Advantages of Lesioning
- Simplicity: Once the lesion is created, there is no need for ongoing management of implanted hardware (as with DBS).
- Cost: Generally less expensive over time than DBS because there is no device and no need for battery replacements or device maintenance.
- Immediate Effect: The therapeutic effect is often noted relatively quickly once the lesion is made.
Disadvantages of Lesioning
- Irreversibility: The procedure permanently destroys brain tissue, so if side effects or complications arise, they cannot typically be reversed.
- Less Flexibility: Unlike DBS, there is no ability to adjust stimulation parameters after surgery.
- Potential for Larger or Off-Target Lesions: If a lesion extends beyond the intended target, it can cause unwanted side effects.
5. Risks and Complications
As with any brain surgery, risks vary depending on the specific target and method, but can include:
- Bleeding (Hemorrhage) within the brain.
- Infection (e.g., at the insertion site in more invasive procedures).
- Neurological Deficits (weakness, changes in sensation, speech difficulties, vision problems, depending on the area targeted).
- Cognitive or Behavioral Changes, especially when targeting structures involved in cognition, emotion, or executive function.
The exact risk profile depends on factors like patient health, surgeon experience, and the target location.
6. Contemporary Trends: Focused Ultrasound
One of the more exciting modern developments is MRI-guided focused ultrasound (FUS):
- Noninvasive: No craniotomy or implanted hardware is required.
- Real-Time Imaging: The procedure is monitored continuously by MRI, allowing precise adjustment of the focal point.
- Fewer Side Effects: Because it is highly focused, surrounding tissue remains largely unaffected.
- Applications: Approved in several countries for medication-refractory essential tremor, and research is ongoing for Parkinsonian tremor, neuropathic pain, and other conditions.
7. Lesioning vs. Deep Brain Stimulation (DBS)
While lesioning surgeries can be effective, DBS has become more popular for conditions such as Parkinson’s disease, essential tremor, and dystonia because:
- Reversibility: DBS can be turned off, adjusted, or removed if side effects or complications occur.
- Customizability: Stimulation parameters can be tailored to a patient’s symptoms and updated over time.
- Bilateral Treatment: In some cases, bilateral lesioning raises higher risk of side effects, whereas DBS can more safely target both sides if needed.
That said, lesioning remains a viable option for certain patients—especially those who might not be good candidates for DBS due to comorbidities, those who cannot manage follow-up programming, or those who want a one-time procedure.
Understanding Different Types of Lesioning Surgery for Brain / Parkinson's Disease / Movement Disorders

1. Radiofrequency (RF) Lesioning
1.1 Methodology
- Stereotactic Placement: A probe is inserted into the target area using stereotactic imaging (CT/MRI) to guide precise placement.
- Tissue Destruction via Heat: Once in place, an electrical current at high frequency (radiofrequency) is applied. The heat generated (often around 60–80°C) destroys the targeted tissue.
1.2 Intended Outcomes & Common Indications
- Movement Disorders: Parkinson’s disease (pallidotomy or subthalamotomy), essential tremor (thalamotomy), dystonia.
- Neuralgia/Pain Syndromes: Trigeminal neuralgia lesioning of the trigeminal nerve root entry zone.
1.3 Benefits
- Immediate Effect: Tissue destruction and symptom relief often occur immediately.
- Relatively Simple and Cost-Effective: No implants or ongoing hardware maintenance are required.
1.4 Risks & Limitations
- Irreversibility: Because it’s an ablative procedure, any adverse effects (e.g., speech or motor deficits) cannot be undone by turning “off” or removing the lesion.
- Precision Required: Off-target lesions can lead to neurological deficits.
- Potential Side Effects: Depending on target, can include weakness, speech difficulties, or other focal deficits.
2. Focused Ultrasound (FUS)
2.1 Methodology
- High-Intensity Ultrasound Beams: Multiple intersecting beams of ultrasound are focused on a single point in the brain, creating enough heat to ablate tissue.
- MRI Guidance: The procedure is typically done inside an MRI scanner to allow real-time temperature monitoring and precise localization.
2.2 Intended Outcomes & Common Indications
- Essential Tremor: FDA-approved in many countries for tremor-dominant cases resistant to medication.
- Parkinsonian Tremor: Being explored, with increasing evidence of efficacy.
- Neuropathic Pain & Other Investigational Uses: Research is ongoing in areas like neuropathic pain, OCD, and depression.
2.3 Benefits
- Noninvasive: No incision or permanent hardware.
- Real-Time Imaging: MRI allows temperature mapping and precise targeting, reducing accidental damage.
- Rapid Recovery: Patients often go home the same day with minimal surgical aftercare.
2.4 Risks & Limitations
- Skull Geometry & Thickness: Not all patients are good candidates because certain skull shapes or densities can deflect or absorb ultrasound energy.
- Thermal Injury: Off-target heating may occur if alignment is imperfect or due to patient movement.
- Irreversibility: As with any lesion, the ablated tissue cannot be restored if side effects develop.
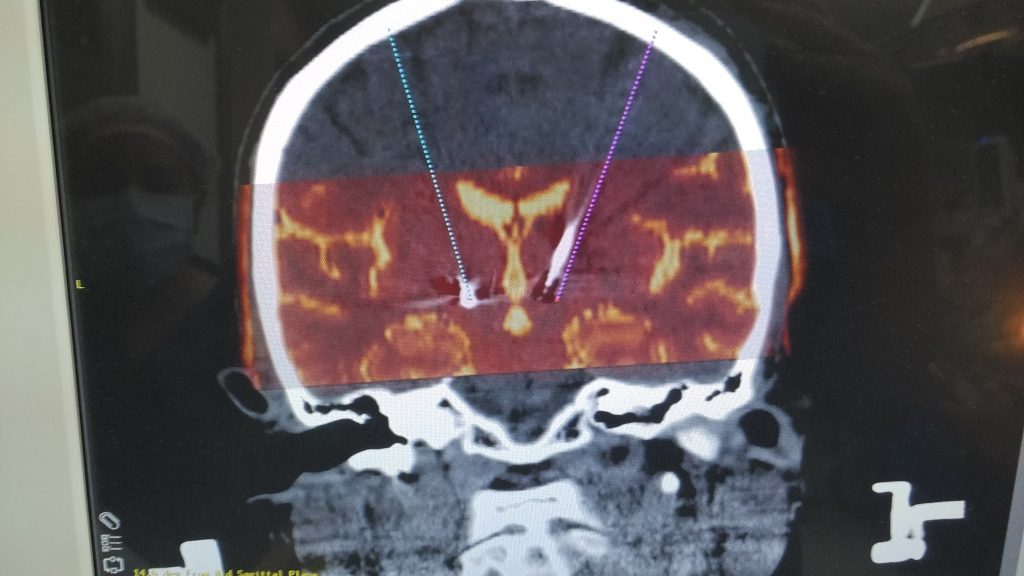
3. Stereotactic Radiosurgery (e.g., Gamma Knife)
3.1 Methodology
- High-Dose Radiation: Concentrated beams of radiation from multiple sources intersect at the target.
- Stereotactic Frame or Mask: Used to stabilize the head, ensuring accurate alignment for radiation delivery.
- Gradual Lesion Formation: Tissue destruction happens over weeks to months as radiation disrupts cellular DNA.
3.2 Intended Outcomes & Common Indications
- Movement Disorders: Thalamotomy for essential tremor or pallidotomy for Parkinson’s in select cases.
- Neuralgia: Trigeminal neuralgia can be treated by targeting the trigeminal root or the trigeminal nerve ganglion.
- Tumors & Vascular Malformations: Though not purely ablative for functional disorders, radiosurgery is widely used for benign tumors like meningiomas, schwannomas, and arteriovenous malformations (AVMs).
3.3 Benefits
- Noninvasive: No open surgery, no hardware implants.
- Precision: Submillimeter accuracy when properly planned.
- Applicable to Some “Deep” Lesions: Areas hard to reach by standard surgery can be targeted with minimal risk of infection or bleeding from an incision.
3.4 Risks & Limitations
- Delayed Onset: Symptom improvement (or lesion formation) may take weeks or months.
- Radiation Risks: Potential for radiation-induced edema or damage to adjacent structures.
- Less Fine-Tuning: Once radiation is delivered, you must wait to see the outcome. You cannot “adjust” it the way you might with deep brain stimulation (DBS).
4. Chemical Lesioning
4.1 Methodology
- Neurotoxic Chemicals: Historically, agents (e.g., alcohol, phenol) were injected stereotactically into targeted brain regions or nerve roots.
- Destruction of Neurons: The chemicals destroy local tissue, interrupting pathological neural circuits.
4.2 Intended Outcomes & Common Indications
- Pain Syndromes: Trigeminal neuralgia, other chronic pain conditions, or spasticity in earlier decades.
- Movement Disorders: Rarely done today for conditions like Parkinson’s disease, given less precision compared to other methods.
4.3 Benefits
- Simplicity & Low Equipment Need: In settings with fewer resources, chemical lesioning may be an option.
4.4 Risks & Limitations
- Poorly Controlled Lesion Size: Harder to precisely define lesion boundaries compared to RF or MRI guidance.
- Potential Spreading: Chemical agents might diffuse unpredictably, damaging unintended areas.
- Largely Obsolete: Supplanted by more sophisticated and safer approaches (radiofrequency, FUS, DBS).
5. Cryoablation (Cryolesioning)
5.1 Methodology
- Extreme Cold: A probe is cooled to very low temperatures (e.g., using liquid nitrogen or argon-based systems) to freeze and destroy targeted tissue.
- Stereotactic Guidance: The probe is placed accurately in the target region.
5.2 Intended Outcomes & Common Indications
- Historically used for:
- Movement Disorders (Parkinson’s disease, essential tremor): Cryothalamotomy or cryopallidotomy.
- Chronic Pain: Some facial pain syndromes or other refractory pain conditions.
5.3 Benefits
- Reduced Risk of Hemorrhage: Cooling can cause local vasoconstriction, possibly reducing bleeding risk during lesion creation.
- Potential for Reversible Test Freeze: In some systems, a brief “test freeze” can be done to check for improvement in symptoms before permanently freezing the tissue.
5.4 Risks & Limitations
- Equipment Complexity: Requires specialized cryosurgery equipment and expertise.
- Less Common Today: Largely replaced by radiofrequency lesioning and DBS.
- Irreversibility: As with other ablative methods, side effects are permanent if mis-targeting occurs.
Comparison of Different Lesioning Surgeries
Comparative Summary
| Technique | Invasiveness | Onset of Effect | Adjustability | Common Uses | Key Advantages | Key Disadvantages |
|---|---|---|---|---|---|---|
| Radiofrequency (RF) | Invasive (Probe) | Immediate | Not adjustable | Parkinson’s (pallidotomy), ET (thalamotomy), trigeminal neuralgia | Immediate effect, relatively simple, cost-effective | Permanent lesion, requires surgical approach, risks of off-target lesion |
| Focused Ultrasound | Noninvasive (no incision) | Near-immediate (during procedure) | Not adjustable | Essential tremor, investigational for PD tremor & neuropathic pain | No incision, real-time MRI guidance, rapid recovery | Skull shape limitations, irreversible, potential off-target heating |
| Radiosurgery (Gamma Knife, etc.) | Noninvasive | Delayed (weeks/months) | Not adjustable | Essential tremor (thalamotomy), trigeminal neuralgia, tumors, AVMs | No incision, high accuracy, minimal infection risk | Delayed effect, radiation risks, irreversible, no real-time feedback |
| Chemical Lesioning | Invasive (Injection) | Relatively quick (hours/days) | Not adjustable | Historically for pain or movement disorders (now rare) | Simple (low-tech) approach in resource-limited settings | Poor lesion control, outdated, risk of chemical spread |
| Cryolesioning | Invasive (Probe) | Immediate after freezing | Partially testable (test freeze) | Historically for movement disorders, pain (less common now) | Potential test freeze, minimal bleeding risk | Specialized equipment, largely replaced by RF & DBS |
Key Takeaways
Key Takeaways
Irreversibility vs. Reversibility
- Lesioning procedures permanently destroy tissue. Any undesirable side effects are usually not correctable post-procedure. This contrasts with Deep Brain Stimulation (DBS), which is adjustable and reversible (by turning off or removing the device).
Invasiveness Spectrum
- Focused Ultrasound (FUS) and Radiosurgery are noninvasive (no incisions).
- Radiofrequency, Chemical, and Cryo lesioning require insertion of a probe/needle or injection, thus are invasive.
Precision and Side-Effect Profile
- MRI-guided Focused Ultrasound is performed with real-time imaging, offering excellent precision. Gamma Knife also provides submillimeter accuracy in many cases.
- Radiofrequency lesioning’s precision depends heavily on intraoperative microelectrode recordings or other physiological mapping.
- Chemical lesioning is the least precise and the most outdated.
Onset of Therapeutic Effect
- Radiofrequency, Focused Ultrasound, and Cryo: The therapeutic effect is often observed during or right after the procedure.
- Radiosurgery: May take weeks or months to see full effect.
Patient Selection
- Lesioning can be an option for patients who are not good candidates for DBS (due to comorbidities, lack of access to long-term DBS programming, or personal preference against implants).
- Focused Ultrasound can be ideal for those who desire a “one-and-done,” noninvasive procedure, if the skull geometry is favorable.
- Radiosurgery is often considered when surgical risk is high (e.g., older patients, bleeding risk), but the delayed onset must be weighed against immediate needs.
Lesioning surgeries span a variety of techniques, each with its own nuances in methodology, outcomes, and risk profiles. While irreversible by nature, they can provide significant symptom relief for movement disorders, pain conditions, and, less commonly, psychiatric disorders (in extremely severe cases). However, modern neurosurgery increasingly leverages Deep Brain Stimulation for reversible and adjustable management, with lesioning reserved for select cases or when DBS is not feasible.
When choosing a lesioning procedure, a thorough evaluation is essential:
- Clinical Indication & Severity: Parkinson’s disease, essential tremor, dystonia, or refractory pain.
- Patient Factors: Overall health, ability to tolerate surgery or follow-ups, skull anatomy (for FUS), preference for noninvasive vs. invasive.
- Long-Term Implications: Understanding that lesioning is not adjustable post-procedure and carries a risk of permanent adverse effects.
Ultimately, the decision to pursue any ablative surgery should involve a multidisciplinary team—neurologists, neurosurgeons, neuropsychologists, and, when appropriate, psychiatrists—to ensure that the approach aligns with the patient’s individual risks, goals, and quality of life considerations.